The healthcare system is designed around objective lab tests for physical health, but mental health lacks similar standardized tools. This absence of objective measures makes...
Latest at the Lab
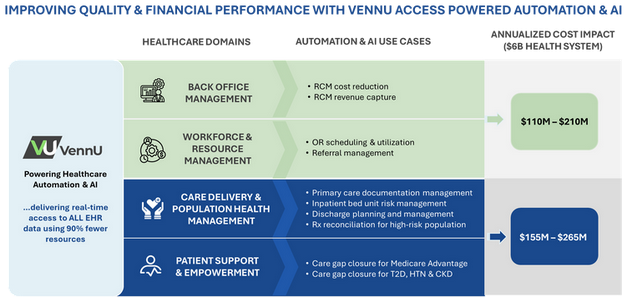
Healthcare systems face the challenge of improving care while controlling costs. Automation and AI offer significant opportunities to enhance both financial and quality outcomes…
Categories
Tags
- Alzheimers
- iFocus
- Vistim Labs
- Delphi
- Cancer
- Breakthrough
- Share
- Dance4Healing
- Suicide
- Compliance
- BuddyAI
- MayPro
- Soliddd
- Macular Degeneration
- Elemrex
- Actual HealthCare Solutions
- Playing Forward
- Lab
- Infant
- RenovaHealth
- Heart
- Mental Health
- Babbly
- White Paper
- Medcurio
- Aging
- AI
- The Proficient Lab
- HelixVM
- Symposia
- Summit
- Spotlight
- Challenge
Healthcare systems face the challenge of improving care while controlling costs. Automation and Artificial Intelligence (AI) offer significant opportunities to enhance both financial and quality...
Heart disease is the leading cause of death for women, yet it remains underrepresented in discussions about women’s health....
Last month was Cervical Cancer month. Cervical cancer is one of the most preventable and treatable cancers when detected early. The traditional Pap smear has...
Last week, HITLAB’s Breakthrough Alliance held a “Share” exclusively for its members. The “Share Session”/ webinar, featured an interview with Robert Garber, Managing Partner at...
HITLAB, a leader in health innovation research, has partnered with Vistim Labs, an emerging pioneer in the evaluation of cognitive and neurodegenerative diseases, to validate...
Laboratory compliance in the life sciences industry is crucial as it directly impacts patient safety, product quality, and data integrity. Pharmaceuticals, medical devices, diagnostics, and...
As we embark on a new year in digital health, HITLAB's leadership team would like to reflect on the significant achievements we've accomplished with our...
HITLAB is thrilled to announce the successful completion of the Fall 2024 semester of its Developing Emerging Leaders in Public Health Innovation (DELPHI) Internship Program....